

Current Projects
- Mormon crickets
- Sex differences in recombination rates
- Speciation
Past Projects
People
- Patrick D. Lorch
- Assistant Professor, Biological Sciences, Kent State University, 256 Cunningham Hall, Kent, OH, 44242-0001, USA
- O: 330-672-7888, F: 330-672-3713
- Email: plorch*at*kent*.*edu
- Publications
- CV (PDF)
- Students
- Applying to work here
Links
Sexual differences in autosomal recombination
Prezi of poster on evolution sex differences in recombination from 2009 SMBE meeting.
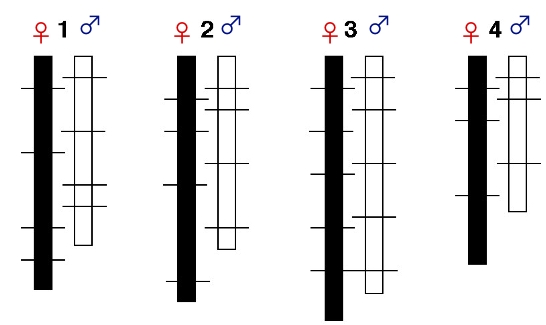
Trivers (1988) highlighted the following pattern:
- There are often sex differences in the rate of
recombination on autosomes as well as on sex chromosomes.
- Typically, recombination is greater in females than in
males and greater in female than male gametic tissue in hermaphrodites.
- Which sex has lower recombination seems to be controlled
by the phenotype.
- Exceptions seem to be explained by situations where sexual selection on males is reduced and may be strong on females (i.e., male parental investment is high, mating is costly for males).
The pattern suggests that there are sex difference in the value of recombination. However, it is not clear whether this pattern exists because males benefit from less recombination or females benefit from more recombination or both. Trivers (1988) proposes an hypothesis that explains this pattern and exceptions to it in terms of males benefitting from reduced recombination. This hypothesis involves sexual selection acting adaptively on groups of genes in one sex. Essentially, individuals of the sex under stronger sexual selection will experience selection for tighter linkage (reduced recombination). This means that there can be conflict between the sexes over recombination rate. It also means that the gametes of the sex experiencing stronger sexual selection are more likely to contain a simple reassortment of parental chromosomes, rather than recombinant versions of the chromosomes from both parents.
An adaptive (non-exclusive) alternative to Trivers' hypothesis is that females benefit from increased recombination rates because it gives them an advantage in sexual conflict over mating frequency. Since in most species males can gain fitness by mating multiple times, the two sexes are expected to have different optimal numbers of mates. Holland and Rice (1998) showed that the conflict generated by this difference in optima can lead males to evolve ways to manipulate females into mating more frequently than is optimal for them. The co-evolutionary race that can ensue may produce a benefit for females of higher rates of recombination than are optimal for males.
Importance
The pattern and Trivers' explanation for it could have important consequences for how we understand evolution at the genetic level. For example, all current models of sexual selection assume equal and constant rates of recombination in each sex (e.g., Higashi et al 1999). Including unequal and evolving recombination rates will likely have dramatic effects on whether these models predict the evolution of exaggerated male display traits and female preferences for these traits. Our understanding of natural selection will most likely also be affected. For example, the unexplained lag (for males relative to females) in the evolution of a cline in male wing length in invading populations of Drosophila subobscura Huey et al (2000) could be due to the fact that males have no recombination. Support for Trivers' explanation of the observed pattern of dimorphism in rates of recombination also raises the following questions:
- Is epistasis more important in one sex while recombination
is more important in the other?
- Understanding the pattern may help us to understand the
evolution of heterogametic sex chromosomes. Why does one sex chromosome
degenerate?
- Haldane (1922) and others Bell (1982) have pointed out
that tight linkage between sex determining factors is expected to
evolve, leading to reduced recombination on the sex chromosomes. This
can lead to Y degeneration and heterogamety (Hamilton 1967).
- An alternative to this is that, as soon as sexes evolve, reduced recombination on all chromosomes in one sex due to sexual selection led to degeneration of one chromosome in the pair bearing the sex determining locus (Bull 1983 and Trivers 1988).
- Haldane (1922) and others Bell (1982) have pointed out
that tight linkage between sex determining factors is expected to
evolve, leading to reduced recombination on the sex chromosomes. This
can lead to Y degeneration and heterogamety (Hamilton 1967).
References
Bell, G. (1982). The Masterpiece of Nature: The Evolution and Genetics of Sexuality. Croom Helm Applied Biology Series. Croom Helm, London, 1 edition.
Bull, J. J. (1983). Evolution of Sex Determining Mechanisms. Benjamin/Cummings, Menlo Park, California.
Haldane, J. B. S. (1922). Sex ratio and unisexual sterility in hybrid animals. Journal of Genetics, 12:101-109.
Hamilton, W. D. (1967). Extraordinary sex ratios. Science, 156:477-488.
Higashi, M., Takimoto, G., and Yamamura, N. (1999). Sympatric speciation by sexual selection. Nature, 402:523-526.
Holland, B. and Rice, W. R. (1998). Chase-away sexual selection: Antagonistic seduction versus resistance. Evolution, 52(1):1-7.
Huey, R. B., Gilchrist, G. W., Carlson, M. L., Berrigan, D., and Serra, L. (2000). Rapid evolution of a geographic cline in size in an introduced fly. Science, 287(5451):308-309.
Rice, W. R. (1996). Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature, 361:232-234.
Trivers, R. L. (1988). Sex differences in rates of recombination and sexual selection. In Michod, R. E. and Levin, B. R., editors, The Evolution of Sex, pages 270-286. Sinauer Associates Inc., Sunderlan, Massachusetts.